HCP ELISA process specific Workflow
Good project preparation in close collaboration with the client is essential for the development of a specific HCP Process. The Agro-Bio team proposes, upstream of the project start-up, to establish precise specifications with the client by organizing several pre-project meetings.
To HCP from ELISA KIT ready-to-use
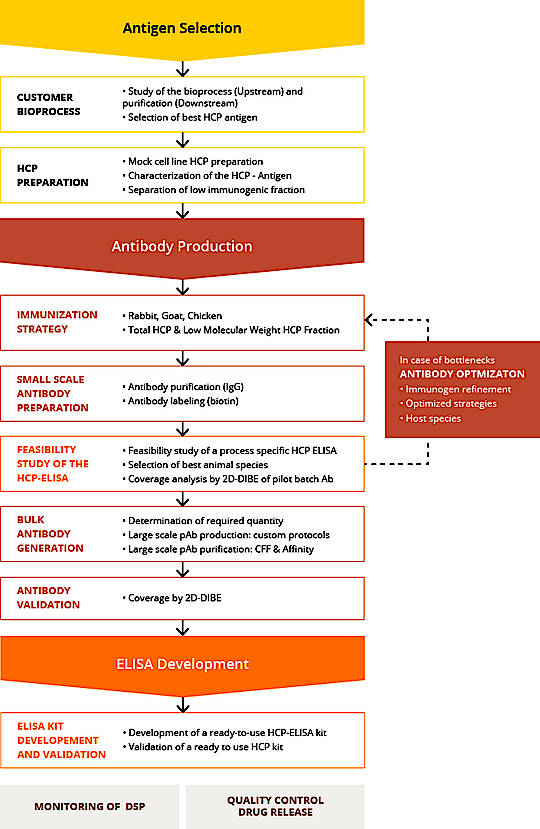
Host Cell Protein (HCP) Antigen Selection
This first step is an essential step in the success of a specific process ELISA HCP development project. Because the goal is to have the sample as the most representative and diverse HCP mix as possible. The Agro-Bio scientific team will be able to support you in order to understand as closely as possible the expression mode of your host cells and how to ideally prepare the sample for the HPP mix.
HCP Antibody Generation
The Agro-Bio animal facility is one of the largest private animal facilities in Europe for the development of antibodies. Thanks to her, Agro-Bio offers a wide range of animal species that can be used in a specific process anti-HCP antibody generation project.
For monitoring this immunization, ELISA, Western Blot, IgG assay controls, etc. will be set up at each crucial step of the immunization protocol, to have a precise analysis of the immune response. They could also be considered as step of “GO / NO GO” that Agro-Bio shares with these customers.
2-D Coverage analysis can be performed at several steps of the protocol and with different matrices: serum, total IgG, immuno-purified IgG. The objective here is to obtain the percentage of coverage defined at the beginning of the project with the client.
For the development of anti-HCP antibodies, all the immunizations, controls, purification, 2D coverage, are processed in-house. In order to guarantee a total confidentiality and great flexibility.
HCP ELISA Development
Once the polyclonal antibodies are fully qualified, the HCP ELISA is set-up.
The preliminary phase to develop a kit consists in the determination of the optimal conditions to stabilize the HCP IgG coated on the ELISA plate. The best coating buffer, IgG concentration and stabilizing conditions will be identified prior to performing an accelerated stability study. Secondary, optimal stabilization conditions for the calibration and control reagents will be assessed to select best conditions and an accelerated stability study will be performed.
The assay can be manufactured as a ready-to-use ELISA kit or the reagents can be transferred to the client or other partner (CDMO, CRO,…) with a standard operating procedure for an in-house implementation.
If required the HCP ELISA Kit Process specific will be validate following the ICHQ2R1 guidelines.

